Introduction
The biotechnology and pharmaceutical sectors are witnessing a remarkable era of innovation in the treatment of metabolic disorders such as obesity and type 2 diabetes. In this landscape, Ascletis Pharma Inc., a clinical-stage biotechnology company focused on innovative therapeutics, has recently announced a significant milestone: the advancement of its novel triple agonist, ASC37, into clinical trials. This development marks a pivotal step in the company’s mission to address the global health burden of obesity and associated diseases through cutting-edge research in hormone receptor modulation.
About Ascletis and Its Therapeutic Vision
Ascletis, headquartered in Hangzhou, China, has long been recognized for its strong R&D pipeline addressing viral, oncological, and metabolic diseases. The company’s strategy lies in building a diversified drug portfolio with both first-in-class and best-in-class drug candidates. By leveraging deep expertise in peptide design and its proprietary platforms, Ascletis has positioned itself at the forefront of metabolic and endocrine drug innovation.
In recent years, the company has expanded its research efforts into obesity and related metabolic disorders — one of the fastest-growing therapeutic areas globally. Obesity, according to the World Health Organization, is associated with increased risks of diabetes, cardiovascular disease, fatty liver, and even certain cancers. The rise in obesity rates underscores the urgent need for more effective and safer therapeutic solutions, which Ascletis is now actively pursuing through its GLP-1R (Glucagon-like Peptide-1 Receptor), GIPR (Glucose-dependent Insulinotropic Polypeptide Receptor), and GCGR (Glucagon Receptor) triple agonist, ASC37.
Understanding the Science: What Is a Triple Agonist?
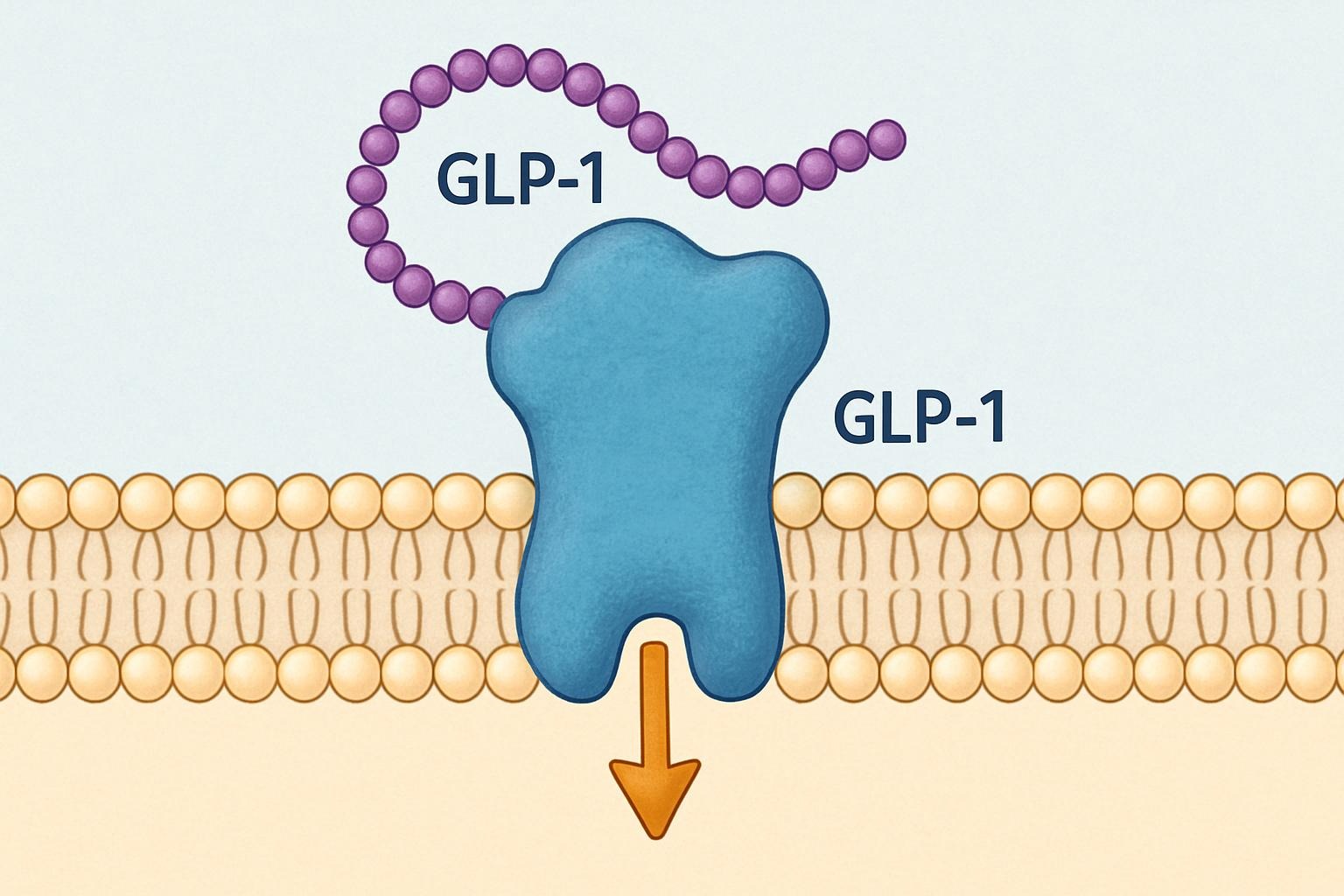
To appreciate the significance of ASC37, it is essential to understand the underlying mechanism of triple agonists targeting GLP-1R, GIPR, and GCGR. These receptors are key regulators of glucose metabolism, appetite control, and energy expenditure.
- GLP-1R (Glucagon-like Peptide-1 Receptor): Stimulates insulin secretion, suppresses appetite, slows gastric emptying, and promotes weight loss.
- GIPR (Glucose-dependent Insulinotropic Polypeptide Receptor): Enhances insulin release in response to glucose intake, improves glucose control, and may support fat metabolism.
- GCGR (Glucagon Receptor): Increases energy expenditure by stimulating glucose production and fat burning, while also helping maintain energy homeostasis.
By combining the actions of these three receptors into a single molecule, ASC37 aims to maximize metabolic benefits — offering robust weight loss, enhanced insulin sensitivity, and improved lipid metabolism — while minimizing adverse effects. This synergistic approach is widely viewed as the next generation of metabolic therapies, building on the success of existing GLP-1R agonists such as semaglutide and tirzepatide.
ASC37: A Next-Generation Triple Agonist
ASC37 stands out as a triple agonist designed with Ascletis’ sophisticated peptide engineering technology. It exhibits high potency and balanced receptor activation, crucial to achieving both efficacy and safety. Early-stage preclinical data have demonstrated promising outcomes in animal models, showing marked reductions in body weight and improvements in glucose tolerance.
Some of the notable attributes of ASC37 include:
- Balanced Activation: Carefully tuned agonist activity across all three receptors to optimize clinical benefit without overstimulation.
- Improved Metabolic Outcomes: Preclinical studies suggest superior effects on glucose control and lipid profiles compared to single or dual agonists.
- Enhanced Safety Profile: Engineered to minimize gastrointestinal adverse effects commonly seen with other GLP-1-based drugs.
- Once-Weekly Dosing Potential: Pharmacokinetic properties support potential weekly administration, improving patient adherence and convenience.
With the transition into clinical development, Ascletis aims to evaluate the safety, tolerability, and pharmacological activity of ASC37 in human subjects, initiating a new chapter in its metabolic drug development pipeline.
The Clinical Trial Milestone
The start of clinical trials for ASC37 represents a critical juncture. According to Ascletis’ announcement, the company has received regulatory clearance in China to commence first-in-human (FIH) studies. The Phase 1 trial will primarily assess:
- Pharmacokinetics (how the drug is absorbed, distributed, metabolized, and excreted).
- Pharmacodynamics (the effects of the drug on metabolism and endocrine markers).
- Safety and tolerability in different dose cohorts.
These preliminary human data will guide subsequent clinical development, including potential Phase 2 studies assessing efficacy in obese and diabetic populations. Given the global competition in GLP-1 and dual/triple agonist therapies, early and positive trial results could position Ascletis among the key innovators in metabolic disease therapeutics.
Global Market Potential and Competitive Landscape
The global obesity treatment market is experiencing exponential growth, driven by the success of GLP-1-based drugs and increasing awareness of obesity as a chronic disease. Market analysts estimate that the segment for incretin-based therapies could surpass $100 billion annually within the next decade.
Ascletis’ ASC37 enters a field currently dominated by:
- Tirzepatide – A dual GLP-1 and GIP receptor agonist developed by Eli Lilly, already approved for obesity and diabetes treatment.
- Retatrutide – Lilly’s triple agonist candidate showing early evidence of remarkable weight loss efficacy.
- CagriSema – Novo Nordisk’s combination therapy built on semaglutide, another GLP-1-based therapy.
However, Ascletis’ entry into the triple agonist category underscores China’s rising presence in global pharmaceutical innovation. By taking a balanced receptor agonism approach and pursuing potential global partnerships, Ascletis could establish ASC37 as a domestic and international contender in the metabolic treatment market.
Strategic Implications for Ascletis
For Ascletis, the advancement of ASC37 is not merely a scientific milestone but a strategic one. The company continues to expand its R&D capabilities, focusing on areas with high unmet medical needs and significant market opportunities. The metabolic drug segment fits seamlessly within its broader vision of developing first-in-class therapies supported by proprietary technologies.
Moreover, this development could:
- Enhance the company’s valuation through strengthening its innovation pipeline.
- Attract new partnerships and investment opportunities from global pharmaceutical companies.
- Reinforce Ascletis’ reputation as a leader in peptide-based drug design and metabolic research.
Ascletis’ multi-pronged business model — encompassing proprietary drug discovery, scale manufacturing, and global collaboration — ensures a robust framework for the future clinical and commercial success of ASC37.
Outlook: Toward the Future of Metabolic Disease Management
The commitment to advancing ASC37 into clinical stages signals a new frontier in obesity and diabetes management. The global healthcare burden of metabolic diseases demands not only effective interventions but those sustainable over the long term. Combining multiple modes of metabolic receptor activation represents a physiological approach to weight management and glucose regulation, addressing both the symptoms and underlying causes of metabolic dysfunction.
If successful, ASC37 has the potential to:
- Deliver superior weight reduction and metabolic benefits compared to monotherapy approaches.
- Reduce reliance on invasive procedures such as bariatric surgery.
- Offer a scalable pharmaceutical solution to populations worldwide struggling with metabolic disorders.
Ascletis’ innovation aligns with a broader trend among biotech firms striving to create combination therapies that address the multifactorial nature of diseases like obesity and type 2 diabetes. The GLP-1 drug revolution is expanding, moving from glucose control to full-spectrum metabolic health optimization — and triple agonists such as ASC37 are at the forefront of this wave.
Conclusion
The initiation of clinical trials for Ascletis’ ASC37 triple agonist marks an important advancement in the treatment landscape for obesity and metabolic disease. By targeting three key hormonal pathways — GLP-1, GIP, and glucagon receptors — the company is pioneering a next-generation therapy designed to deliver holistic metabolic benefits.
As the world seeks sustainable, safe, and effective treatments for chronic metabolic conditions, Ascletis’ scientific progress offers both hope and momentum. With ASC37 entering clinical evaluation, the company positions itself strategically among global leaders in the metabolic therapeutic frontier.
In the coming years, the outcomes of these trials will determine whether ASC37 can translate preclinical promise into real-world clinical success, shaping the future of obesity and diabetes management worldwide.